Patients For Stem Cells (PFSC) is a group of patients faced with no-option medical conditions. Many of us have been early adopters of stem cell therapy, and are only here today because it saved our lives. Frustrated by slow adoption in the U.S, we advocate for expedited access. We are very pleased to share with our readers that 2015 has brought watershed changes in legislation that lays the foundation to implement this goal. Leaders in the regenerative medicine field have outlined how access can become a reality. Implementation in on the horizon, and will be in focus at this landmark conference.
The Alliance for the Advancement of Cellular Therapies (AACT), organizer of this event, is a welcome new ally, the bridge between patients, government and regenerative medicine leaders who can finally make real change happen. ACCT is dedicated to the ethical, efficacious, and expeditious advancement of cell therapies.
To help spread awareness and education about cellular therapy, AACT can send a personal conference invite to your doctor, send a request to info@AACT.co

Patients are core stakeholders of ACCT, thanks to President and Co-Founder Kathy Hebert.Her personal experience with life-changing cellular therapies ignited her passion to bring everyone together to achieve the best possible patient outcomes.
For the conference, Kathy has brought together a group of presenters, keynoted by Arnold Caplan,Ph.D. Mark Holterman, MD. Ph.D.ACCT Chairman and Co-Founder, and Paolo Macchiarini, MD, Ph.D

Keynote Speakers
Leading up to the conference we will profile the contributions these luminaries have already made to the lives of patients, as architects of accelerated access, and best in-class clinicians utilizing cell therapy to address difficult disease such as heart failure, auto immune disease, Alzheimer’s, Parkinson’s, osteoarthritis, and more.
Presenters will also include those involved in legislation that swept the nation as 22 states passed “Right To Try” laws for terminally ill patients to access experimental therapies, including biologics like stem cells. Then on July 10th, 2015 the 21st Century Cures Act was approved by the U.S. House of Representatives, also addressing ways to speed regenerative medicine access. Now is the time for us to dive into the details of all these initiatives, and make sure our best interests as patients are met.
A special thank you to Dr. Camillo Ricordi for publishing the Proceedings of the AACT conference in
We encourage you to join the discussion in social media and give us your feedback!
AACT Facebook Twitter Google+ LinkedIn AACT.co
PFSC Facebook Twitter
Want to become a member of Patients For Stem Cells? Just subscribe to our blog!






 For the first time in history the FDA has encroached into the practice of medicine. The FDA made autologous adult stem cell therapy subject to the same regulatory oversight as mass-produced pharmaceutical agents. Without compliance to the Administrative Procedures Act requiring an “extensive public comment period.“ The FDA managed to expand it’s authority, without input from patients and their doctors…
For the first time in history the FDA has encroached into the practice of medicine. The FDA made autologous adult stem cell therapy subject to the same regulatory oversight as mass-produced pharmaceutical agents. Without compliance to the Administrative Procedures Act requiring an “extensive public comment period.“ The FDA managed to expand it’s authority, without input from patients and their doctors…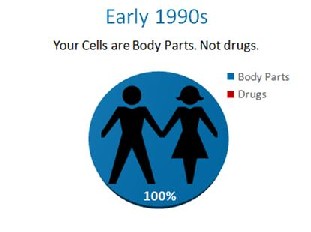 In the early 1990’s, the cells in your body were like any other body part that could be used by a physician and moved from one area to the next. They were body parts like a kidney or a heart. That was before an unprecedented power grab by the FDA.
In the early 1990’s, the cells in your body were like any other body part that could be used by a physician and moved from one area to the next. They were body parts like a kidney or a heart. That was before an unprecedented power grab by the FDA. 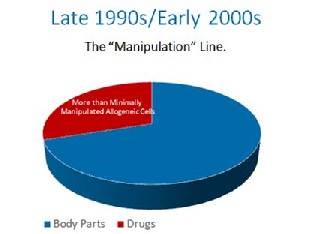 In the late 1990s, the FDA proposed that the cells in your body should be made drugs. They were met with stiff resistance. They ultimately decided, that if the cells were at all made more potent by growing them to bigger numbers, they would be drugs, even though they were still your cells. This was known as the “minimal manipulation” rule later codified in 21 CFR 1271.1-3. In the 1990’s this only applied to someone else’s cells that were manufactured like drugs, so this made some sense.
In the late 1990s, the FDA proposed that the cells in your body should be made drugs. They were met with stiff resistance. They ultimately decided, that if the cells were at all made more potent by growing them to bigger numbers, they would be drugs, even though they were still your cells. This was known as the “minimal manipulation” rule later codified in 21 CFR 1271.1-3. In the 1990’s this only applied to someone else’s cells that were manufactured like drugs, so this made some sense. 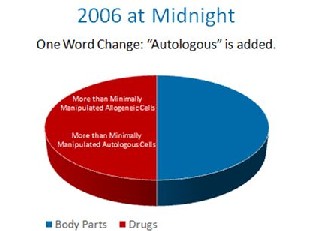 The FDA, without the proper notice and comment making period required by the Administrative Procedures Act, made a one word midnight change in the 2006 federal register. It changed a single word from “another” to “a”. By doing this, it’s regulatory authority was expanded from simple control over someone’s cells used as a transplant from “another” person to all cells from “a” person. With this one work change, FDA granted itself sweeping new authority over your body.
The FDA, without the proper notice and comment making period required by the Administrative Procedures Act, made a one word midnight change in the 2006 federal register. It changed a single word from “another” to “a”. By doing this, it’s regulatory authority was expanded from simple control over someone’s cells used as a transplant from “another” person to all cells from “a” person. With this one work change, FDA granted itself sweeping new authority over your body. 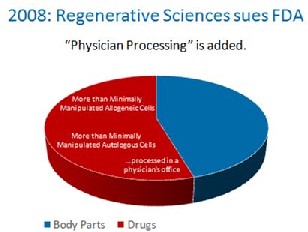 Up until 2008, it was assumed that FDA only meant to apply it’s rules to pharma companies who were processing cells. Then in 2008, FDA went after a physician’s office that was using the patients own cells and treated the small doctor’s office like a large Pharma company. This necessitated a suit by the doctor’s office against FDA, but this chain of events now extended FDA’s authority even further, as now your local family doctor was suddenly under the same cell based regulations as Pfizer.
Up until 2008, it was assumed that FDA only meant to apply it’s rules to pharma companies who were processing cells. Then in 2008, FDA went after a physician’s office that was using the patients own cells and treated the small doctor’s office like a large Pharma company. This necessitated a suit by the doctor’s office against FDA, but this chain of events now extended FDA’s authority even further, as now your local family doctor was suddenly under the same cell based regulations as Pfizer. 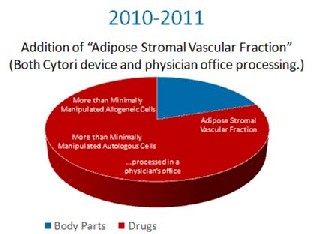 In 2010 and 2011, the FDA decided to place fat tissue processed at the patient’s bedside to release stem cells into the same category as prescription drugs. They made this intention clear through letters to several parties that asked the Tissue Reference Group whether this simple processing of fat was something FDA intended to control. In addition, it submitted Warning Letters to Intellicell and a Dr. Young, codifying it’s intent to turn fat tissue into a drug.
In 2010 and 2011, the FDA decided to place fat tissue processed at the patient’s bedside to release stem cells into the same category as prescription drugs. They made this intention clear through letters to several parties that asked the Tissue Reference Group whether this simple processing of fat was something FDA intended to control. In addition, it submitted Warning Letters to Intellicell and a Dr. Young, codifying it’s intent to turn fat tissue into a drug.  So as you can see, FDA has gone from no control over your body’s stem cells as drugs in the 1990’s, to classifying an ever increasing number of you body tissues as drugs. This is despite massive opposition from these industry groups.
So as you can see, FDA has gone from no control over your body’s stem cells as drugs in the 1990’s, to classifying an ever increasing number of you body tissues as drugs. This is despite massive opposition from these industry groups.  Share this page with your friends. Ask your local university if they have a focus on adult stem cell research, specifically autologous mesenchymal stem cells, expanded to a therapeutic dose. Educate your state and congressional representatives. Citizens need to take charge of the way their body parts are being regulated.
Share this page with your friends. Ask your local university if they have a focus on adult stem cell research, specifically autologous mesenchymal stem cells, expanded to a therapeutic dose. Educate your state and congressional representatives. Citizens need to take charge of the way their body parts are being regulated.